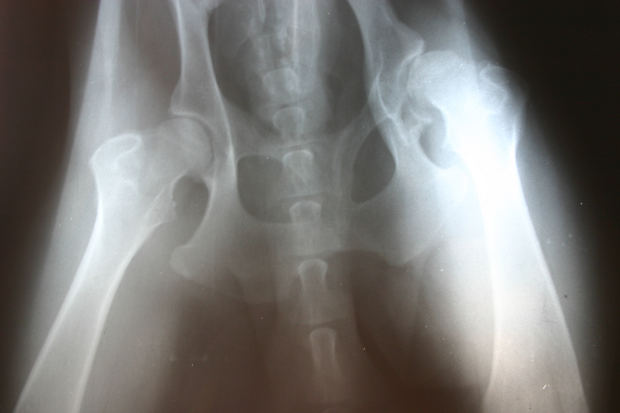
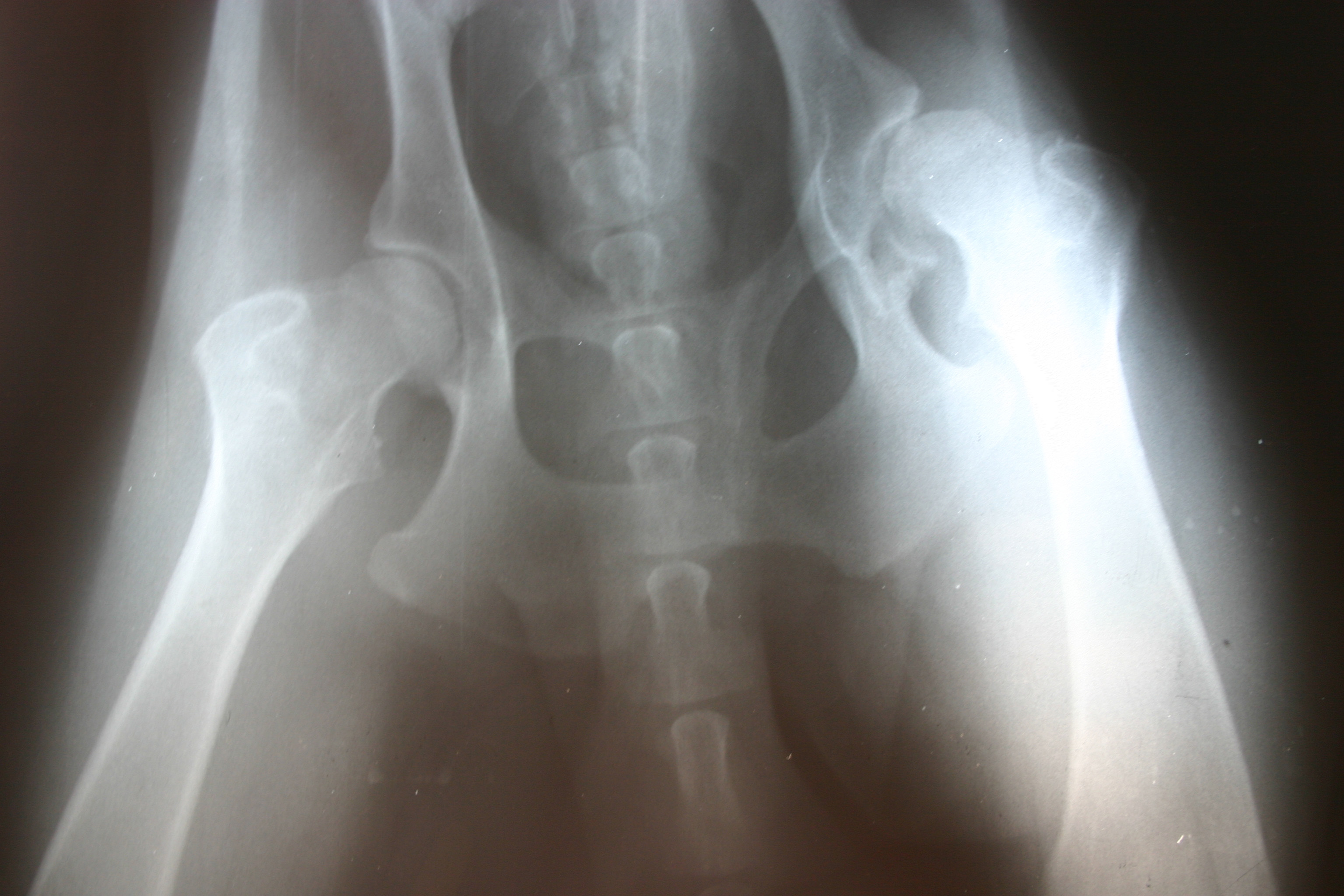
Recent case studies in leading medical journals indicate that patients with metal hip joint replacements could be at a greater risk of cardiac problems and complications. According to a New York Times article, two recent investigations uncovered similar medical mysteries, both involving patients with metal hip replacements and life-threatening heart conditions. The medical research and reports could help future patients with hip replacements and cardiac problems identify the source of their illness or disease. Our Boston hip implant lawyers are dedicated to recent developments involving health and dangerous medical devices.
Case study 1: A man in Germany became ill and sought a diagnosis to treat a worsening condition. His medical problems had begun three years earlier, including low hormone levels, an inflamed esophagus, and high body temperature. He began to lose his vision to the point of being nearly blind. The patient had damaged hearing and became nearly deaf. His illness intensified and he felt his body weaken, evidently because of a heart condition. Eventually, he suffered heart failure, even though he had no coronary artery disease or complications.
Continue reading
 Product Liability Lawyer Blog
Product Liability Lawyer Blog