Massachusetts continues to see meaningful movement in both verdicts and device litigation.
Big Massachusetts & New England Headlines
Record asbestos verdict underscores long-tail exposure claims.
Massachusetts continues to see meaningful movement in both verdicts and device litigation.
Big Massachusetts & New England Headlines
Record asbestos verdict underscores long-tail exposure claims.
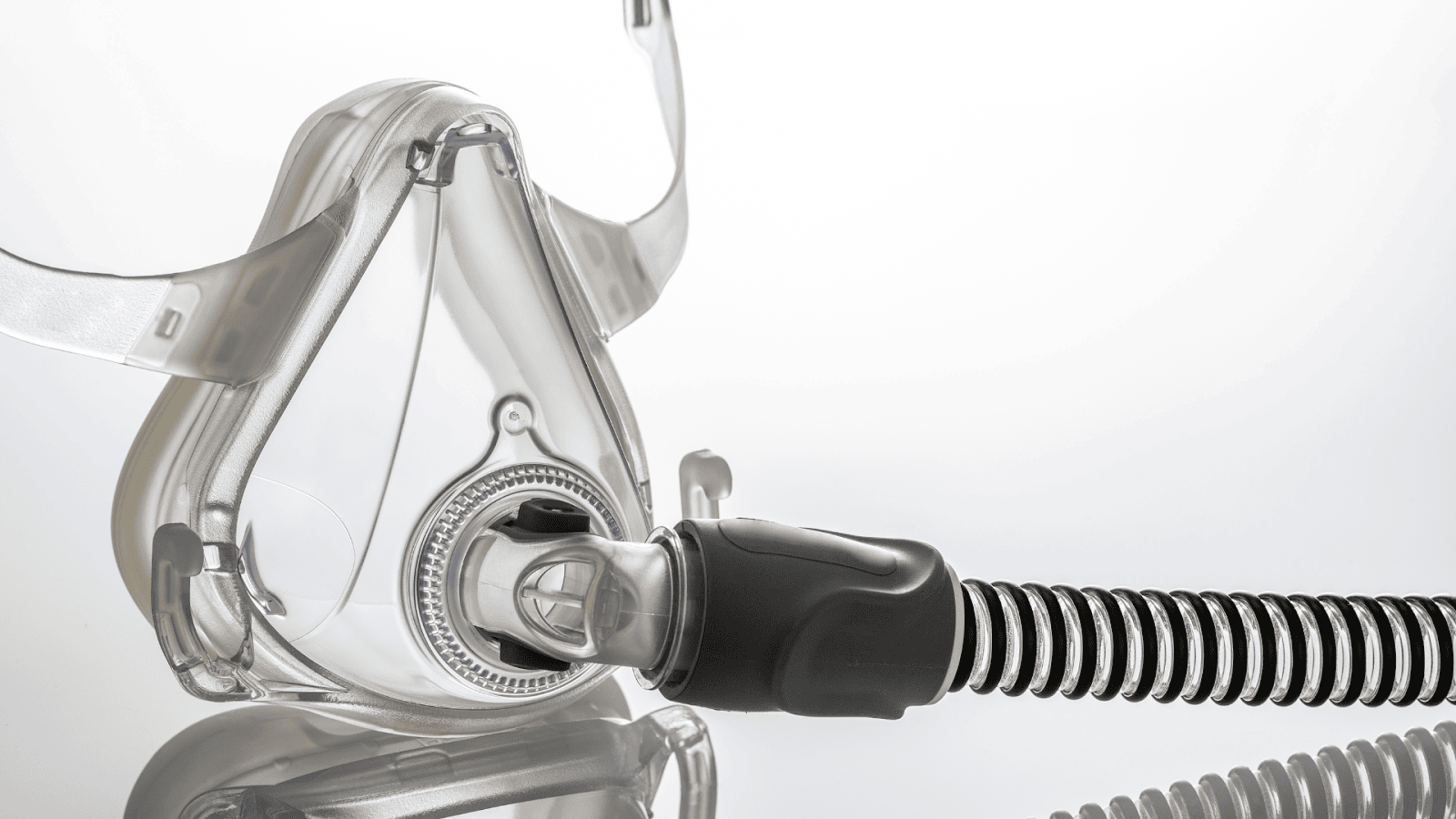
In an unprecedented move that has caught the attention of healthcare providers and patients alike, Philips Respironics issued a recall for specific models of its BiPAP and CPAP machines in 2021. This recall raises significant concerns for individuals relying on these devices for respiratory support, marking a critical juncture in the realm of patient care and medical device reliability. The announcement has sent ripples through the medical community, prompting a reevaluation of the safety and efficacy of such essential respiratory support devices. Recognizing the gravity of this situation, Jeffrey Glassman Injury Lawyers is committed to advocating for the rights and well-being of our clients.
Important Update on Philips Respironics Machine Recalls
The U.S. Food and Drug Administration (FDA) has issued critical updates on the recall of specific Philips Respironics CPAP, BiPAP, and ventilator machines, highlighting the serious nature of the situation with 561 deaths associated with these devices since the recall began in 2021. These machines, essential for individuals with sleep apnea and other respiratory conditions, have been identified as having significant issues that could pose severe health risks to users. The alarming number of associated fatalities underscores the urgency for affected individuals to take appropriate actions and seek guidance.
The device can break and perforate internal organs
When you purchase birth control, you should be able to trust that it’s safe for use. Unfortunately, that’s not always the case, as the attorneys at Jeffrey Glassman Injury Lawyers can confirm.
always the case, as the attorneys at Jeffrey Glassman Injury Lawyers can confirm.
One form of birth control that has been making headlines in recent months is a copper IUD called Paragard that has been around for decades. In fact, the Paragard website touts that the device is the only IUD on the market that has had U.S. Food and Drug Administration approval for more than 30 years.
When it comes to health care in the United States, it’s consumer beware.
A look at medical device and pharmaceutical drug recalls issued by the U.S. Food & Drug Administration in 2018 illustrates the consequences of a lax regulatory and approval process run largely by the drug companies and medical device manufacturers: A primary focus of the watchdog agency task with the nation’s safety must be warning consumers about the dozens of dangerous drugs and medical devices that it has approved for market.
Experienced Massachusetts product liability attorneys know too well that the nation’s healthcare system is a primary offender when it comes to consumer harm caused by dangerous or defective products.
A products liability lawsuit involves an injury caused by a dangerous or defective product. This can involve dangerous drugs like Xarelto, or defective artificial hips like the Pinnacle ASR metal-on-metal hip manufactured by DePuy, or it can involve seemingly harmless household products that possess hidden dangers. There are various factors that will determine if a company is liable for a product that caused an injury including the following:

There is no question that medical devices and durable medical equipment are big business. There is also a lot of competition and these medical device firms are trying to get as many new products on the market as possible to maximize profits. While that can result in a lot of good for patients in need of new treatments, The rush to market can also result in shortcuts being taken, and these shortcuts are often taken at the expense of patients’ health and safety.
 One of the reasons they are able to get these new devices on the market so quickly is because of a loophole of sorts in the medical device regulations governed by the U.S. Food and Drug Administration (FDA). If there is a similar device already on the market, and there often is, the company may be able to skip certain testing and then go back and do post-market testing. Continue reading
One of the reasons they are able to get these new devices on the market so quickly is because of a loophole of sorts in the medical device regulations governed by the U.S. Food and Drug Administration (FDA). If there is a similar device already on the market, and there often is, the company may be able to skip certain testing and then go back and do post-market testing. Continue reading
There are various types of claims that can be filed in a civil tort lawsuit. Briefly, according to the Second Restatement of Torts, a tort is an act or omission that gives rise to injury or harm to another and amounts to a civil wrong for which the courts may impose liability. This definition is the one used by judges and is taught to every law student in America. Essentially, this definition means that when someone does something that causes an injury to another person, and this is the type of conduct for which the court will impose a remedy, a case can be filed on this basis.
 There are various different types of torts, and there are also what are known as intentional torts and negligence torts. For the purposes of personal injury law, we are mainly talking about negligence torts, though, there can also be a lawsuit for intentional torts like assault and battery, false imprisonment (civil kidnapping), arson, conversation (theft) and various others. Continue reading
There are various different types of torts, and there are also what are known as intentional torts and negligence torts. For the purposes of personal injury law, we are mainly talking about negligence torts, though, there can also be a lawsuit for intentional torts like assault and battery, false imprisonment (civil kidnapping), arson, conversation (theft) and various others. Continue reading
According to a recent news article from NASDAQ, Medtronic has begun to voluntarily recall all unused units of their StrataMR product lines. StrataMR is a line of adjustable valves and shunts that patients and doctors have been complaining about lately. These complaints range from minor issues to potentially deadly adverse events. One person has died, but the company has yet to determine the exact cause of the death.
The medical devices are used to treat a medical condition known as hydrocephalus, which involves the buildup of fluid on a patient’s brain. If this fluid is allowed to build up, it presses against the inner layer of skull known as the dura, and this creates a tremendous amount of pressure. If untreated, this can result in permanent brain injury or even death. Continue reading
Lawsuits against Atrium Medical Corp. for its C-QUR hernia mesh product alleging these devices were not safe for use by humans. 
One of the most recent cases is one filed in Florida Middle District Court in Orlando just this past month. In his filing, which alleges negligent manufacturing and strict product liability, plaintiff alleges he underwent surgery in April 2013 to repair multiple hernias. His surgeon used the C-QUR product. Subsequently, plaintiff alleges, he suffered injury.
Although the recent Florida Record article reporting the filing does not offer specific details on the man’s injury, we can make the inference based on previous claims, the type of liability asserted here and also what we know about the company’s history. Continue reading
When you go into a hospital on the day of your surgery, you have likely met with your surgeon and discussed the risk and benefits of the benefits of whatever procedure you are scheduled to have that day, assuming you are not brought into the emergency room and rushed into an operating room. You are likely confident that surgeon has the skill and experience to do his or her job properly, and while this is very likely true, if your surgeon is unknowingly using a defectively-designed medical device, the chance of a complication may be exceedingly high.
 In a case from the Washington State Supreme Court, a surgeon who had used a defendant’s surgical assist robot on numerous occasions spoke to the patient about his intention to use the relatively new device to operate on the patient’s prostate cancer. The doctor obtained informed consent from the patient, who was considered severely obese at the time of his surgery. Continue reading
In a case from the Washington State Supreme Court, a surgeon who had used a defendant’s surgical assist robot on numerous occasions spoke to the patient about his intention to use the relatively new device to operate on the patient’s prostate cancer. The doctor obtained informed consent from the patient, who was considered severely obese at the time of his surgery. Continue reading