Our Boston hip replacement attorneys understand that representing a client injured by one of the now-recalled DePuy artificial hips requires a great deal of familiarity with defective medical product litigation. These can be extremely complicated cases.
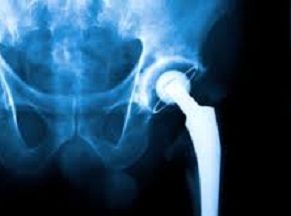 According to a recent article, the State of Oregon has settled with DePuy Orthopedics for $4 million over deceptive marketing involving artificial hips.
According to a recent article, the State of Oregon has settled with DePuy Orthopedics for $4 million over deceptive marketing involving artificial hips.
This case involved a metal-on-metal artificial hip that was prone to failing. It has been established that the metal would deteriorate over time, producing shards of metal that have caused muscle damage and other complications requiring surgeries. The iron itself has been known to cause damage to the liver, kidneys, and other internal organs.
Continue reading
 Product Liability Lawyer Blog
Product Liability Lawyer Blog