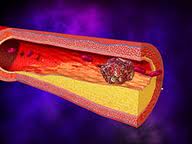
As an increasing number of Americans are faced with the difficult decision of whether to pursue hip or knee replacements, an increasing number of cases involving complications. This has given rise to hundreds of thousands of lawsuits, millions in medical expenses, and ongoing health issues for patients. Now Bristol-Meyers Squibb has announced that the U.S. Food and Drug Administration has approved a new supplemental drug for deep vain thrombosis, which could result in pulmonary embolism in patients who have undergone hip or knee replacement surgery.
The new drug, known as Eliquis is intended to prevent deep pain thrombosis in patients who have undergone hip or knee replacement. Advocates for the drug claim that the approval is a significant milestone for the medication and for the patients who suffer from additional health risks associated with knee and hip implants. Our Boston products liability attorneys are dedicated to helping victims or defective or dangerous products recover just compensation for their injuries. In addition to pursuing financial claims, we are committed to raising awareness surrounding the complications associated with medical device injury.
According to a medical news report, a spokesperson for Bristol-Meyers Squibb sees the drug as a breakthrough in providing new treatment options for patients and their physicians. Since the number of patients seeking implants is on the rise, so is the number of patients who are at risk of deem vein thrombosis or pulmonary embolism. Unfortunately, the risks associated with hip and knee replacement and the injuries associated with the medical devices go beyond the risk of deep vein thrombosis.
There are an estimated 600,000 knee implants every year and a growing number of replacements in younger patients. Advocates for these patients, including lawyers and doctors, are seeing a rising trend in the number of complications and failures after replacement. There are a host of manufacturers and devices and each pose a different set of challenges and risks. In addition to the health risks posted to patients, taxpayers may also be forced to cover the excessive costs of treatment and surgery.
In addition to deep vein thrombosis, patients also report complications involving infection and joint dislocation. For patients who suffer from extreme complications, treatment may require revision surgeries or, for some patients, multiple revision surgeries over a lifespan. Manufacturers have been held liable for introducing products without proper testing. Often problems are not identified until they are tested on patients and then recalled. Some implants have not yet been recalled, despite their known dangers and risks to patients.
The FDA approval of Eliquis may reduce the risk of stroke or deep vein thrombosis (DVT) in some patients. DVT is usually caused by a blood clot in a large vein, usually in the lower leg, thigh or pelvis. If the clot travels to the lung or a blood vessel causing a block, the victim could suffer sudden death. Patients of knee or hip implants are also at risk of fractures, loosening of device, dislocation, wear and tear, stiffness and infection. If you or someone you love has suffered complications related to a knee or hip implant, an experienced advocate can protect your rights.
Call Jeffrey Glassman Injury Lawyers for a free and confidential appointment — (617) 777-7777.
More Blog Entries:
$1.2 Million Vaginal Mesh Verdict for Plaintiff,Boston Product Liability Lawyer Blog, April 8, 2014
TVM Company Facing Falling Revenues & Legal Trouble, Boston Product Liability Lawyer Blog, March 13, 2014
 Product Liability Lawyer Blog
Product Liability Lawyer Blog